A new function of flavivirus NS2B in virion assembly is uncovered
Date:02-06-2016 | 【Print】 【close】
Flavivirus nonstructural protein 2B (NS2B) is a transmembrane protein that functions as a cofactor for viral NS3 protease. The cytoplasmic region (amino acids 51-95) alone of NS2B is sufficient for the NS3 protease activity, whereas the role of transmembrane domains (TMDs) remains obscure. The Research Group of Flavivirus Infection, led by Prof. Bo Zhang demonstrates for the first time, that flavivirus NS2B plays a critical role in virion assembly.
Using Japanese encephalitis virus (JEV) as a model, they performed a systematic mutagenesis at the flavivirus-conserved residues within the TMDs of NS2B. As expected, some mutations severely attenuated (L38A and R101A) or completely (G12L) destroyed viral RNA synthesis. Interestingly, two mutations (G37L and P112A) reduced viral RNA synthesis and blocked virion assembly. None of the mutations affected the NS2B-NS3 protease activity. Because mutations G37L and P112A affected virion assembly, they selected revertant viruses for these two mutants. For mutant G37L, a substitution with G37F, G37H, G37T, or G37S restored virion assembly. For mutant P112A, an insertion of K at position K127 (leading to K127KK) of NS2B rescued virion assembly. A biomolecular fluorescent complementation (BiFC) analysis demonstrated: (i) mutation P112A selectively weakened the NS2B/NS2A interaction; (ii) the adaptive mutation K127KK restored the NS2B/NS2A interaction. Collectively, their results demonstrate that, besides as a cofactor for NS3 protease, flavivirus NS2B also functions in viral RNA replication as well as virion assembly.
Many flaviviruses are important human pathogens. Understanding the molecular mechanisms of viral infection cycle is essential for vaccine and antiviral development. In this study, the research group demonstrates that the TMDs of JEV NS2B participate in both viral RNA replication and virion assembly. Viral genetic study and BiFC assay demonstrated that interaction between NS2B and NS2A may participate in modulating viral assembly in flavivirus life cycle. Compensatory mutation analysis confirmed that there was a correlation between viral assembly and NS2B/NS2A interaction. TMDs of NS2B may serve as a novel antiviral target to prevent flavivirus infection and the structure determination of NS2B will help to understand the function mechanism of NS2B in viral RNA replication and assembly. The results have uncovered a new function of flavivirus NS2B in virion assembly, possibly through interaction with the NS2A protein.
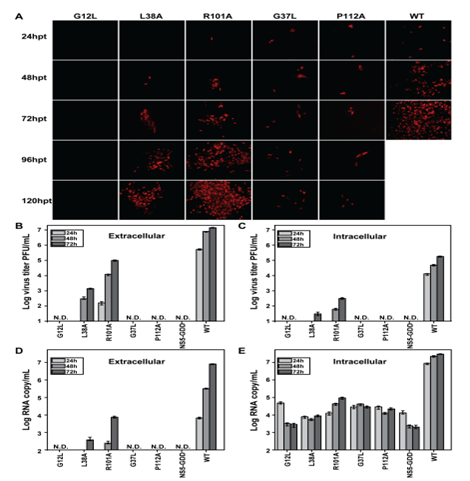
Characterizations of the phenotypes of NS2B mutants in the context of778 JEV infectious clone.