A new research report appearing on August 30th 2016 in Cell Reports dissects the cellular role of WhiB6, one of the WhiB redox sensor family proteins, in virulence and intracellular survival of pathogenic mycobacteria, according to researchers from the Center for Emerging Infectious Diseases, Wuhan Institute of Virology (WIV), Chinese Academy of Sciences (CAS).
As one of the most successful intracellular pathogens, mycobacterium tuberculosis (Mtb) causes 8 million cases of tuberculosis and 1.3 million deaths worldwide annually. During the course of infection, Mtb is exposed to diverse redox stresses that trigger metabolic and physiological changes. However, it remains unclear how these stressors are sensed and relayed to the Mtb transcriptional apparatus. Researchers have already known that the ESX-1 secretion system encoding a type VII secretion system is unique to mycobacteria and is required for acute infection, while the dosRS regulon is required for long-term persistence in Mtb. Furthermore, association of nitric oxide (NO) produced by host cells and upregulation of dosR as well as whiB6 has been documented, but how this happens remains to be elucidated.
“Using the M. marinum-zebrafish infection model, we provide compelling evidence showing that WhiB6 acts as a finely tuned regulator of the ESX-1 secretion system and dosR regulon with its Fe-S cluster in response to NO”, according to CHEN Zhenkang, a Ph.D. student and the first author of this paper.
As Mtb infection aggravates, infected macrophages activate additional macrophages and other immune cells to form a granuloma, which is an organized collection of macrophages composed of mononuclear phagocytes, dendritic cells, as well as T and B lymphocytes. “Our study reveals that WhiB6 regulation has altered function due to change toward its Fe-S cluster, which enables mycobacteria to establish persistent infection and maintain integrity of the granulomas. We propose a model (see below) to explain how WhiB6 plays in different regulatory roles to modulate the development of granulomas”, said CHEN Shiyun, Ph.D., a principal investigator and the corresponding author of this paper. “Our work is of great interest not only to the specific field of mycobacteriology, but also to the broader readership interested in host-pathogen interaction and related mechanisms.”
The study, “Mycobacterial WhiB6 differentially regulates ESX-1 and the Dos regulon to modulate granuloma formation and virulence in zebrafish”, is supported by grants from CAS Key Program and National Natural Science Foundation of China (NSFC). Additional authors include Bridgette Cumming and Adrie Steyn from KwaZulu-Natal Research Institute for Tuberculosis and HIV, South Africa.
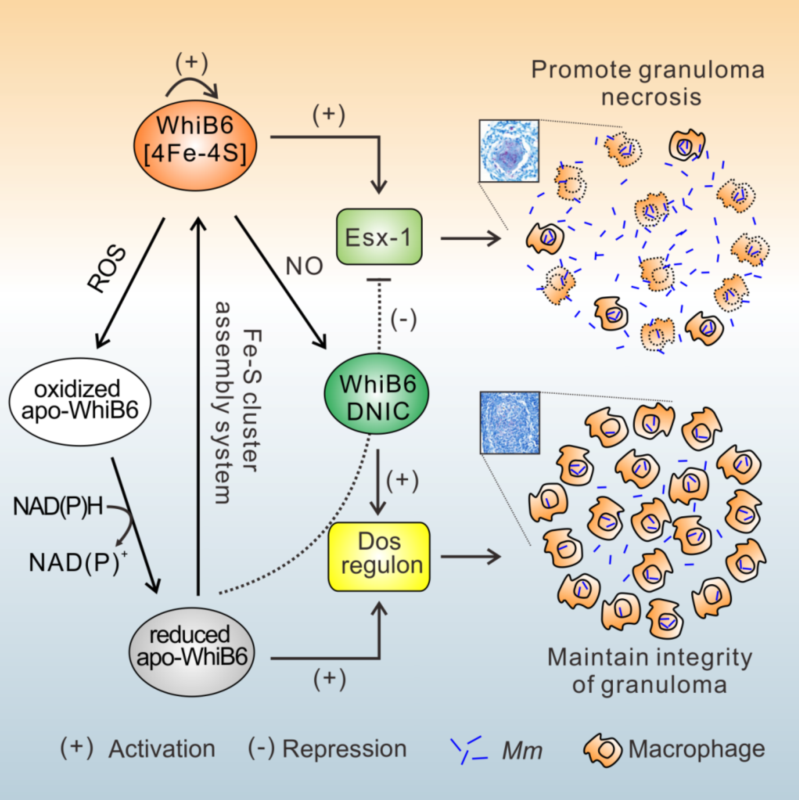
A proposed model for WhiB6-mediated modulation of disease dissemination and maintenance of granulomas. (Image by CHEN Shiyun)
Contact:
Prof. CHEN Shiyun, PH.D., Principle Investigator
Wuhan Institute of Virology, Chinese Academy of Sciences
Wuhan, Hubei 430071, China
Tel: 86-027-87199354
E-mail: sychen@wh.iov.cn