Scientists in WIV reveal the asymmetric movement of the double-stranded RNA
Date:25-12-2016 | 【Print】 【close】
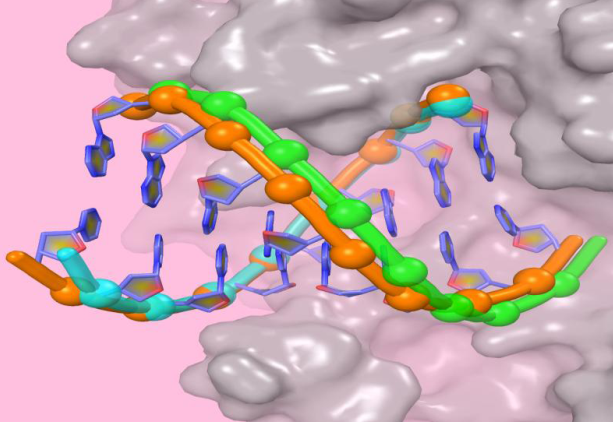
RNA viruses encode a unique class of RNA-dependent RNA polymerases (RdRPs) to carry out their fully RNA-based genome replication and transcription. Although the chemical nature of nucleotide addition is essentially shared by all nucleic acid polymerases, the structural and mechanistic details taken by each polymerase class differ to various extents. The research group led by Prof. Peng Gong in WIV reports seven crystal structures of enterovirus 71 RdRP elongation complex at 2.5–2.8 Å resolution. In these structures the polymerases are poised at various distinct stages to reveal mechanistic details of initial NTP binding, key amino acid side-chain conformational switches during active site closure, and inparticular the postcatalysis movement of the RNA duplex on the way to vacate the active site for the next nucleotide addition cycle.
Viral RNA-dependent RNA polymerases (RdRPs) play essential roles in viral genome replication and transcription. The research group previously reported several structural states of the poliovirus RdRP nucleotide addition cycle (NAC) that revealed a unique palm domain-based active site closure mechanism and proposed a six-state NAC model including a hypothetical state representing translocation intermediates. Using the RdRP from another human enterovirus, enterovirus 71, here they report seven RdRP elongation complex structures derived from a crystal lattice that allows three NAC events. These structures suggested a key order of events in initial NTP binding and NTP-induced active site closure and revealed a bona fide translocation intermediate featuring asymmetric movement of the template–product duplex. Their work provides essential missing links in understanding NTP recognition and translocation mechanisms in viral RdRPs and emphasizes the uniqueness of the viral RdRPs compared with other processive polymerases.
Link: http://www.pnas.org/content/113/28/E4005.long