SUMO Modification Stabilizes Enterovirus 71 Polymerase 3D to Facilitate Viral Replication
Date:25-12-2016 | 【Print】 【close】
Accumulating evidences suggest that viruses hijack cellular proteins to circumvent the host immune system. Ubiquitination and SUMOylation are extensively studied post-translational modifications (PTMs) that play critical roles in diverse biological processes. Crosstalk between ubiquitination and SUMOylation of both host and viral proteins has been reported to result in distinct functional conses1quences. Enterovirus 71 (EV71), an RNA virus belonging to Picornaviridae family, is a common cause of hand, foot and mouth disease. Little is known concerning how host PTM systems interact with enteroviruses.
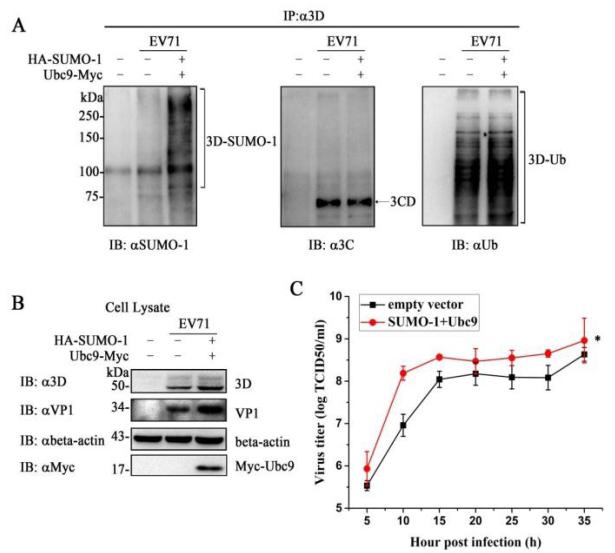
Under this circumstance, Prof. Hanzhong Wang and his research group in WIV demonstrated that the 3D protein, an RNA-dependent RNA polymerase (RdRp) of EV71, is modified by small ubiquitin-like modifier-1 both during infection and in vitro. Residues K159 and L150/D151/L152 were responsible for 3D SUMOylation determined by bioinformatic prediction combined with site-directed mutagenesis. And primer-dependent polymerase assays indicated that mutation of SUMOylation sites impaired 3D polymerase activity and virus replication. Moreover, 3D is ubiquitinated in a SUMO-dependent manner, and SUMOylation is crucial for 3D stability which may be due to the interplay between the two PTMs. Of importance, increasing the level of SUMO-1 in EV71-infected cells augmented the SUMOylation and ubiquitination level of 3D, leading to enhanced replication of EV71. These results together suggested that SUMO and ubiquitin cooperatively regulated EV71 infection either by SUMO-ubiquitin hybrid chains or by ubiquitin conjugating to the exposed lysine residue through SUMOylation. Their study provides a new insight into how a virus utilizes cellular pathways to facilitate its replication.
Link: http://jvi.asm.org/content/early/2016/09/08/JVI.01756-16.abstract