Herpes simplex virus (HSV) is a prevalent worldwide human pathogen that infects epithelial cells before it establishes latency in trigeminal or sacral nerve root ganglia, causing mucocutaneous lesions, keratitis, and encephalitis. The development of vaccines and novel therapeutic strategies against HSV has become very urgent.
Glycoprotein D (gD) is the most abundant glycoprotein on the virion and the major stimulus for virus-neutralizing antibodies of HSV. For both vaccine design and novel therapeutic strategies, it is important to study epitopes on gD that stimulate virus-neutralizing antibodies.
In a present study led by Prof. WANG Hualin in Wuhan Institute of Virology of Chinese Academy of Sciences, the scientists found a novel monoclonal antibody (mAb), m27f, targeting to glycoprotein D (gD) of HSV-2, which also has cross-reactivity against HSV-1 gD. M27f was found to recognize a new continuous epitope (residues 292 to 297) within the pro-fusion domain of HSV and possesses a high level of virus-neutralizing activity. It showed a high degree of neutralizing activity against both HSV-1 and HSV-2, completely abrogated viral cell-to-cell spread, and inhibited syncytium formation in vitro. In addition, it also exhibited highly therapeutic effects in a HSV-2 infected mouse model, implying its high potential for adaptation as protective or therapeutic interventions.
In conclusion, their results have demonstrated for the first time that mAb m27f targeting a new continuous epitope (residues 292 to 297) within the pro-fusion domain has a high level of virus-neutralizing activity. These finding will enrich the HSV glycoprotein D-specific neutralizing antibodies and will facilitate the development of vaccine design or novel therapeutic strategies.
The results have been published in Antiviral Research entitled "A novel glycoproteinD-specific monoclonal antibody neutralizes herpes simplex virus".
This work was supported by the National Science Foundation of China; the National Key Research and Development Program from the Ministry of Science and Technology of China; and the European Union's Horizon 2020 EVAg project.
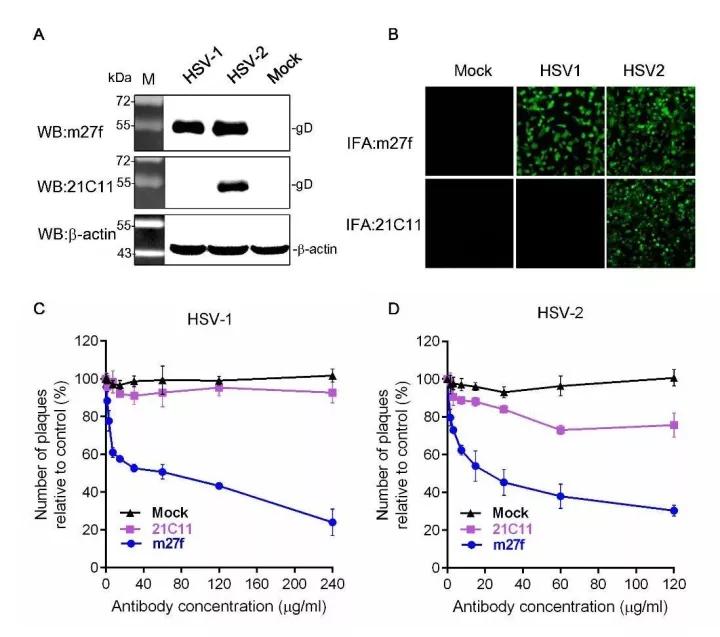
Figure1. Characterization of mAbs m27f and 21C11. Image by WANG Hualin

Figure 2. m27f blocks cell-to-cell spread of HSV-1 and HSV-2. Image by WANG Hualin
Contact:
WANG Hualin
E-mail: h.wang@wh.iov.cn
Wuhan Institute of Virology, Chinese Academy of Sciences, Wuhan 430071, China (http://english.whiov.cas.cn/)