Prion protein (PrP) is a glycosylphosphatidylinositol anchored protein widely expressed in central nervous system, lymphoreticular system and GI tract. It has been attributed to neurodegenerative diseases such as prion disease and alzheimers disease. However, normal functions of PrP remains in-completely understood.
Recently, more and more papers reported that PrP is up-regulated in many types of cancers and contributes to tumorigenesis. In the cases of pancreatic ductal adenocarcinoma, breast cancer, and gastric cancer, expression of PrP is the biomarker for poor prognosis of the patients.
New research carried out by scientists at the Center for Molecular Virology, Wuhan Institute of Virology of the Chinese Academy of Sciences, investigated the role of PrP in melanoma and pancreatic cancer tumorigenesis.
Their study was published online on September 12th in Journal of Biological Chemistry entitled “Prion protein is required for tumor necrosis factor α (TNFα)-triggered nuclear factor κB (NF-κB) signaling and cytokine production”.
“Working with M2 melanoma cell line and BxPC-3 pancreatic ductal adenocarcinoma cell line, we successfully deleted PRNP gene using Crispr/Cas9 technique. Treatment of PrP expressing and PrP null M2 cells with TNFα revealed that only M2 cells expressing PrP showed TNF receptor (TNFR) response, resulting in NFκb signaling. To prove that there is functional interplay between PrP and TNFR, we also down regulated PrP expression with siRNA or we rescued the PrP expression in PrP null M2 cells by transducing PrP expression, we found indeed that PrP is required for the activation of NFκb signaling”, said WU Guiru, the first author of the paper.
“To identify the mechanism of PrP regulating NFκb signaling, we performed co-immunoprecipitation of PrP with monoclonal antibody specific for PrP. We found that CYLD, a de-ubiquitinase that co-purified with PrP. In addition, treatment with TNFα enhanced the co-location and co-purification of PrP and CYLD, suggesting that PrP by pulling CYLD away from TNFR complex stimulated NFκb signaling. Indeed, we found that interaction between CYLD and RIP1 or interaction between CYLD and Traf2 were reduced only when PrP was present and only when the cells were treated with TNFα. Accordingly, we found that TNFα treatment resulted in significantly reduce poly-ubiquitinated RIP1 or Traf2 in the absence of PrP compared to when PrP is present”, Ms. WU added.
“Based on our results, we propose a model to explain how PrP responds to TNFα to enhance tumorigenesis”, said Dr. LI Chaoyang, a principal investigator and the corresponding author of the paper.
“Our work is of great interest not only to the specific field of prion biology, but also to the broader readership interested in cancer biology”, he added.
The study was supported by grants from the National Natural Science Foundation of China, by Strategic Priority Research Program A of the Chinese Academy of Sciences, and by Ministry of Science and Technology of China.
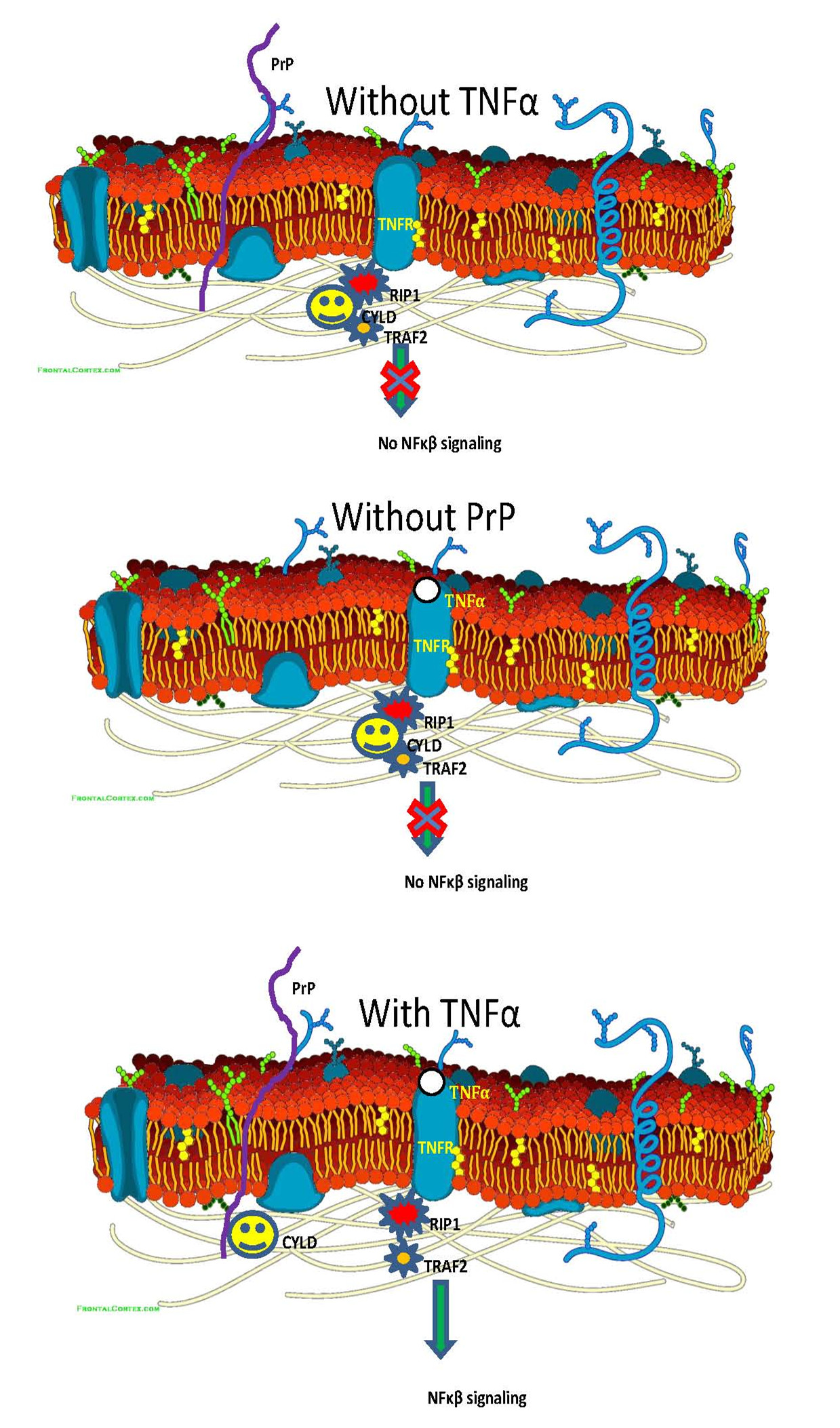
PrP is required for the transcriptional activity of NF-κB and production of TNFα. Image by LI Chaoyang
Contact:
LI Chaoyang
E-mail: cyli@wh.iov.cn
Wuhan Institute of Virology, Chinese Academy of Sciences, Wuhan 430071, China (http://english.whiov.cas.cn/)
