Scientists show that lacidipine is a candidate for LASV therapy
Date:22-06-2018 | 【Print】 【close】
Lassa virus (LASV) is an enveloped, negative-sense, bi-segmented RNA virus belonging to the Mammarenavirus genus (family Arenaviridae). To date, no vaccines or specific antiviral agents against LASV are available. Therapy strategies are limited to the administration of ribavirin in the early course of the illness.
To address this issue, the research group led by Prof. XIAO Gengfu in Wuhan Institute of Virology of the Chinese Academy of Sciences, shows that lacidipine is a candidate for LASV therapy, reinforcing the notion that the SSP-GP2 interface provides an entry-targeted platform for arenavirus inhibitors design.
In this study, the scientists screened an FDA-approved drug library of 1018 compounds. The approved drugs have been intensively investigated for safety, pharmacokinetics, and targets; therefore, screening approved drugs for repurposing will increase the speed of discovery and development for treatment. Drugs targeting viral entry can block replication and spread at an early stage.
Since studies of LASV require BSL-4 equipment, the scientisrs utilized a LASV GPC pseudotype vesicular stomatitis virus (VSV) containing a Renilla luciferase (Rluc) reporter gene for high-throughput screening (HTS) of LASV entry inhibitors, which can be performed in a BSL-2 facility. After three rounds of screening, lacidipine and phenothrin were identified to be highly effective against LASV entry. The hit compounds identified in this study offer potential new therapies to treat arenavirus infections and disease.
The results have been published in Journal of Virology entitled "Screening and Identification of Lassa Virus Entry Inhibitors from an FDA-Approved Drugs Library".
This work was supported by the National Key Research and Development Program of China, the National Natural Sciences Foundation of China, the Open Research Fund Program of CAS Key Laboratory of Special Pathogens and Biosafety, Wuhan Institute of Virology, and the Open Research Fund Program of Wuhan National Bio-Safety Level 4 Lab of CAS, the Open Research Fund Program of the State Key Laboratory of Virology of China.
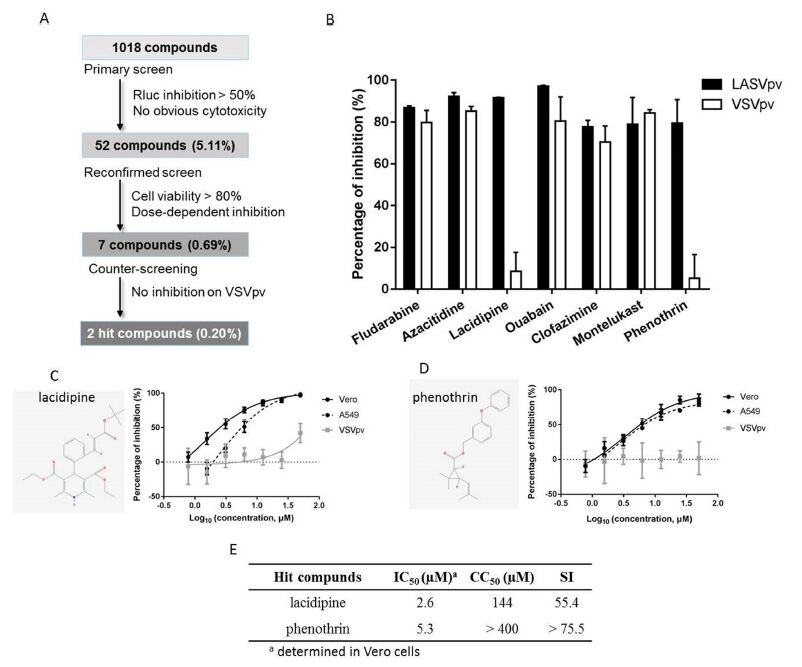
Figure 1. High throughput screening (HTS) for inhibitors of Lassa virus (LASV) entry from a Food and Drug Administration (FDA)-approved drug library. Image by XIAO Gengfu
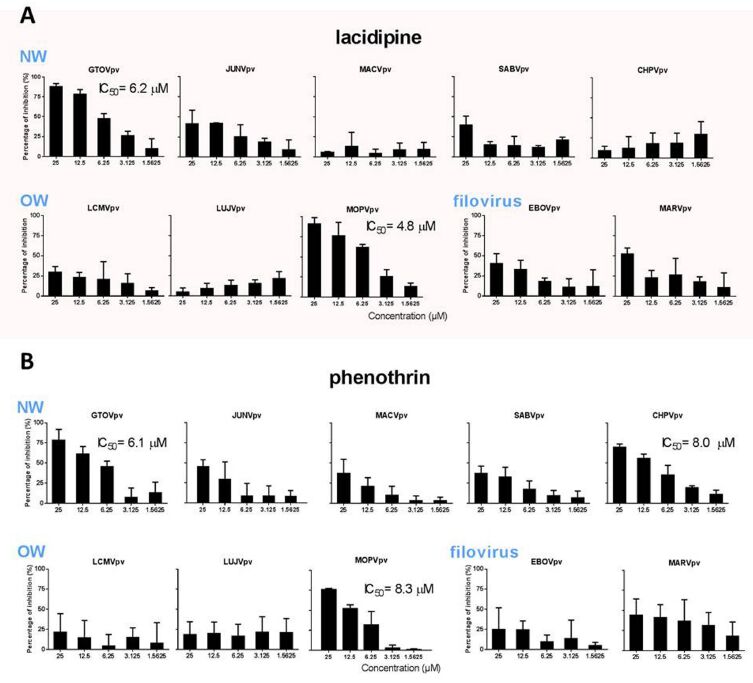
Figure 2. Broad spectrum antiviral activity of the hit compounds against different mammarenavirus and filovirus. Image by XIAO Gengfu
Contact:
XIAO Gengfu
E-mail: xiaogf@wh.iov.cn
Wuhan Institute of Virology, Chinese Academy of Sciences, Wuhan 430071, China (http://english.whiov.cas.cn/)