Scientists constructed three human IgG1 Fc mutants for better clinical performance
Fc-based therapeutic proteins include therapeutic antibodies and Fc-fusion proteins. Therapeutic monoclonal antibodies (mAbs) with high affinity and specificity are now widely used in treatment of cancer, immune disorders, viral infection and other diseases. Fc-fusion proteins are also emerging as promising biopharmaceuticals because of the additional benefits from Fc fragment.
Unfortunately, both mAbs and Fc-fusion proteins might be unstable and susceptible to aggregation, which may not meet the requirements for therapy in vivo. Therefore, in the field of the development of recombinant therapeutic proteins, control and prevention of unfolding and aggregation are essential for ensuring efficacy and safety.
As the common part of all the full-size mAbs and Fc-fusion proteins, Fc fragment is an attractive target for optimization. In a present study, three Fc mutants with additional disulfide bonds in CH2 domain, CH3 domain and both CH2 and CH3 domains were designed by the research group led by Prof. GONG Rui in Wuhan Institute of Virology of Chinese Academy of Sciences.
A series of experiments were performed for comparison of stability, aggregation resistance and function of these Fc mutants with wildtype Fc. The roles of engineered disulfide bonds in different Fc domains to the stability, aggregation resistance and function have been comprehensively elucidated. The most stable and aggregation-resistant mutants in CH2 and CH3 domains with reservation of Fc-mediated function could be used for modification of Fc-based therapeutics toward better clinical outcomes.
This study gives a comprehensive elucidation of structural and functional effects caused by additional disulfide bonds in the Fc fragment, which is important for Fc engineering toward the desired clinical performance.
The results have been published in the Journal of Biological Chemistry entitled "Comprehensive elucidation of the structural and functional roles of engineered disulfide bonds in antibody Fc fragment".
This work was supported by Key Program of Chinese Academy of Sciences Grant (ZDRW-ZS-2016-4), National Key Research and Development Program of China Grant (2016YFC1202902), National Natural Science Foundation of China Young Scientists Program Grant (81302690), and Novo Nordisk–Chinese Academy of Sciences Research Fund Grant (NNCAS-2014-10).
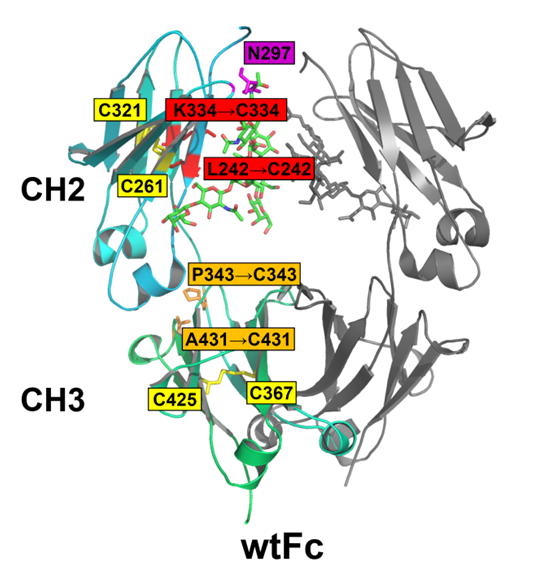
Schematic of design of Fc mutants with additional disulfide bonds. Image by GONG Rui
Contact:
GONG Rui
E-mail: gongr@wh.iov.cn
Wuhan Institute of Virology, Chinese Academy of Sciences, Wuhan 430071, China (http://english.whiov.cas.cn/)
