Cytomegalovirus encodes a protein to reach the balance between anti-apoptosis and immune evasion
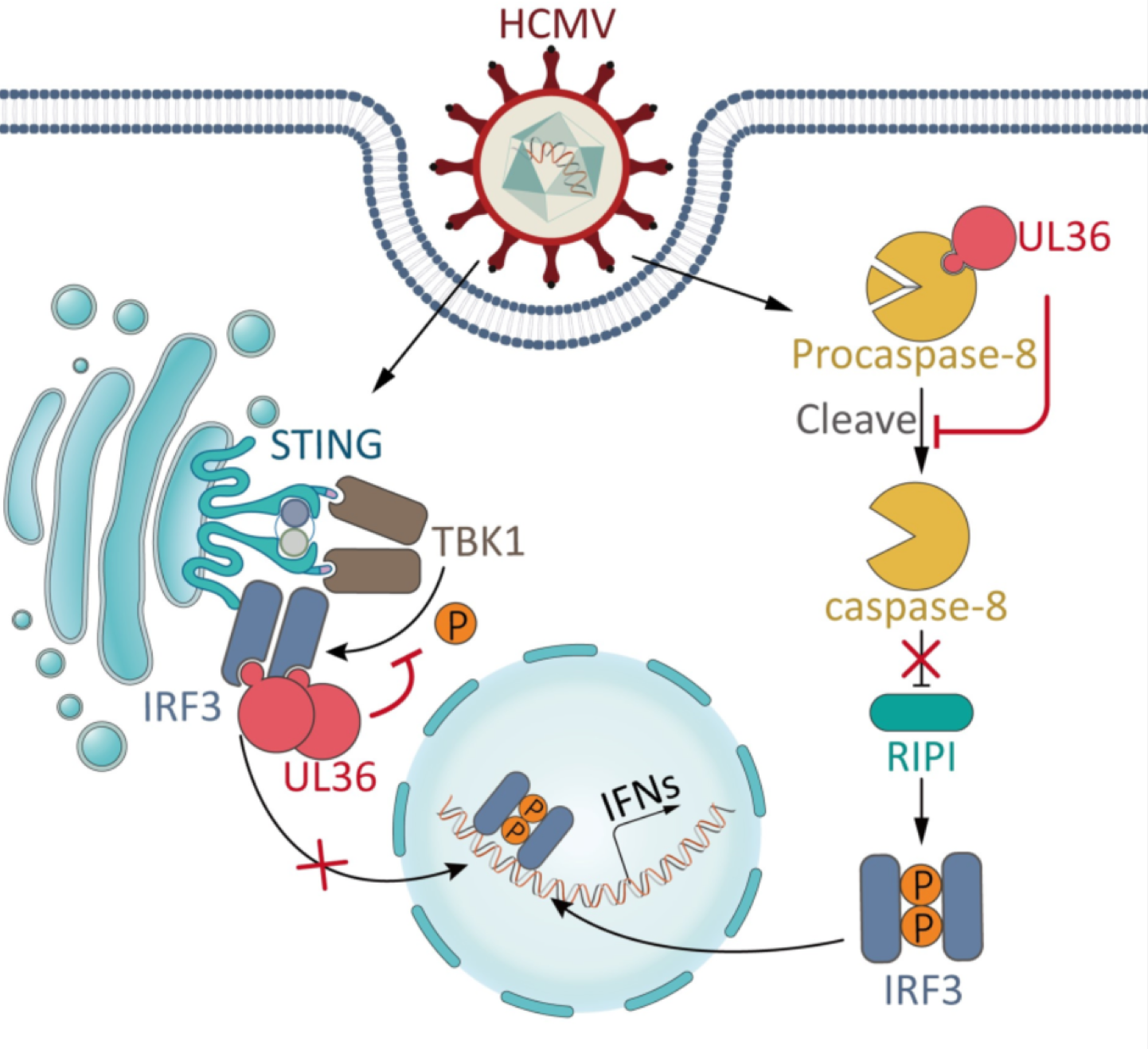
Human cytomegalovirus (HCMV) is a β-herpesvirus that infects most of the global population and causes serious threats to human health. Moreover, the constant exposure of HCMV to innate immune system makes this virus to evolve a rich arsenal of immune evasion strategies. In addition to innate immunity, apoptosis also acts as an important antiviral mechanism by removing infected cells. Thus, inhibition of apoptosis is also of critical importance for HCMV. Among HCMV-encoded proteins, UL36 has long been identified as an inhibitor of extrinsic apoptotic pathway by directly binding and inhibiting caspase-8. However, previous study reported that caspase-8 can inhibit IRF3 activation. Therefore, although caspase-8/apoptotic inhibition by HCMV-encoded UL36 can provide an advantageous cellular environment for HCMV infection, it would inevitably result in enhanced antiviral immune signaling that is detrimental for this virus. Such a “side-effect” brings a dilemma of how to balance immune evasion and anti-apoptosis, the two pivotal requirements for infection by HCMV and other viruses encoding apoptotic inhibitors.
In this study, the research group led by Prof. ZHOU Xi from Wuhan Institute of Virology, Chinese Academy of Sciences uncovered that in addition to inhibiting caspase-8/extrinsic apoptosis, HCMV UL36 antagonizes IRF3-dependent immune signaling by directly targeting IRF3. UL36 simultaneously confers these two inhibitory functions during HCMV infection. While inhibiting caspase-8/extrinsic apoptosis by UL36 enhances immune signaling, the immunosuppressing activity of UL36 counterbalances this immunoenhancing “side-effect” for viruses, and the dual inhibition by UL36 has particularly functional importance for HCMV life cycle.
It is worth to note that a previous study from the same group uncovered that another HCMV protein, UL37x1, also confers dual inhibition on both apoptosis and innate immunity (Ren et al. Nat Microbiol. 7, 1041-53, 2022). Different from UL36, UL37x1 is an inhibitor of intrinsic apoptosis, while UL37x1 inhibits immunity by targeting TBK1. Therefore, the same virus encodes two distinct proteins, UL36 and UL37x1, to respectively inhibit extrinsic and intrinsic apoptosis, which pinpoint the critical value to establishing a sophisticated regulatory network to inhibit both extrinsic and intrinsic apoptotic pathways as well as their corresponding immunoenhancing effects, thereby ensuring a balanced, favorable cellular microenvironment for HCMV. Given that most viruses (not limited to herpesviruses) employ their own strategies to inhibit apoptosis, it would be intriguing to find out how these viruses reach such a balance between immune evasion and anti-apoptosis.