Scientists reveal the significant impact of assembly morphology on the immunogenicity of viral capsid proteins
Date:30-05-2024 | 【Print】 【close】
In this study, the research group led by Prof. Feng LI from the Wuhan Institute of Virology of the Chinese Academy of Sciences (CAS), in collaboration with colleagues, employed the VLPs of the MS2 phage as a model to investigate the shape?function relationship of protein nanostructures in terms of serving as an immunogen. With the aid of molecular dynamics simulations, they redesigned the critical region of the assembly interface, FG loop, and mutated residue 70 to cysteine (C70), which mediated the formation of disulfide bonds, disrupting the natural icosahedral symmetry while guiding building blocks (CP dimers) to form one-dimensional structures. As a result, the C70 mutation, in combination with the mutation of cysteine 46 to alanine (to exclude potential interference from the endogenous cysteine 46), completely transformed the spherical nanocage structure of MS2 VLPs into ultrafine double-helical nanotubes, with an average length of approximately 1 μm, an outer diameter of 8.6 nm, and an inner diameter of 2.6 nm. The secondary and tertiary structures of the mutant protein were almost identical to those of the wild type but with differences in the flexibility of the FG loop. The mutant building blocks formed a completely different arrangement pattern from the wild type. Interestingly, mutating residue 70 to amino acids with hydrophobic side chains also led to forming similar nanotubes, while mutating to histidine resulted in single-helical nanotubes. Furthermore, introducing cysteine at residue 75 on the nanotube surface triggered crosslinking to form reductant-responsive hydrogels (Figure 1). In virtue of the tunable self-assembly system, they compared the immunogenicity of MS2 CPs in three different forms, nanocages, nanotubes, and nanotube gels, showing that in comparison with nanocages, nanotubes significantly enhanced both humoral and cellular immune responses, while hydrogels significantly boosted the levels of cytokine secretion and cellular cytotoxicity of CD8+ T cell (Figure 2).
This work has achieved a hierarchically transformable system of viral capsids and revealed the significant impact of assembly morphology on the immunogenicity of viral CPs, providing new insights for the design of nanovaccines and other functional protein nanomaterials.
The study has been published in ACS Nano and is entitled “Transformation of a Viral Capsid from Nanocages to Nanotubes and Then to Hydrogels: Redirected Self-Assembly and Effects on Immunogenicity.” This work was supported by the National Key Research and Development Program, the National Natural Science Foundation of China, the Science and Technology Program of Wuhan, and the Knowledge Innovation Program of Wuhan.
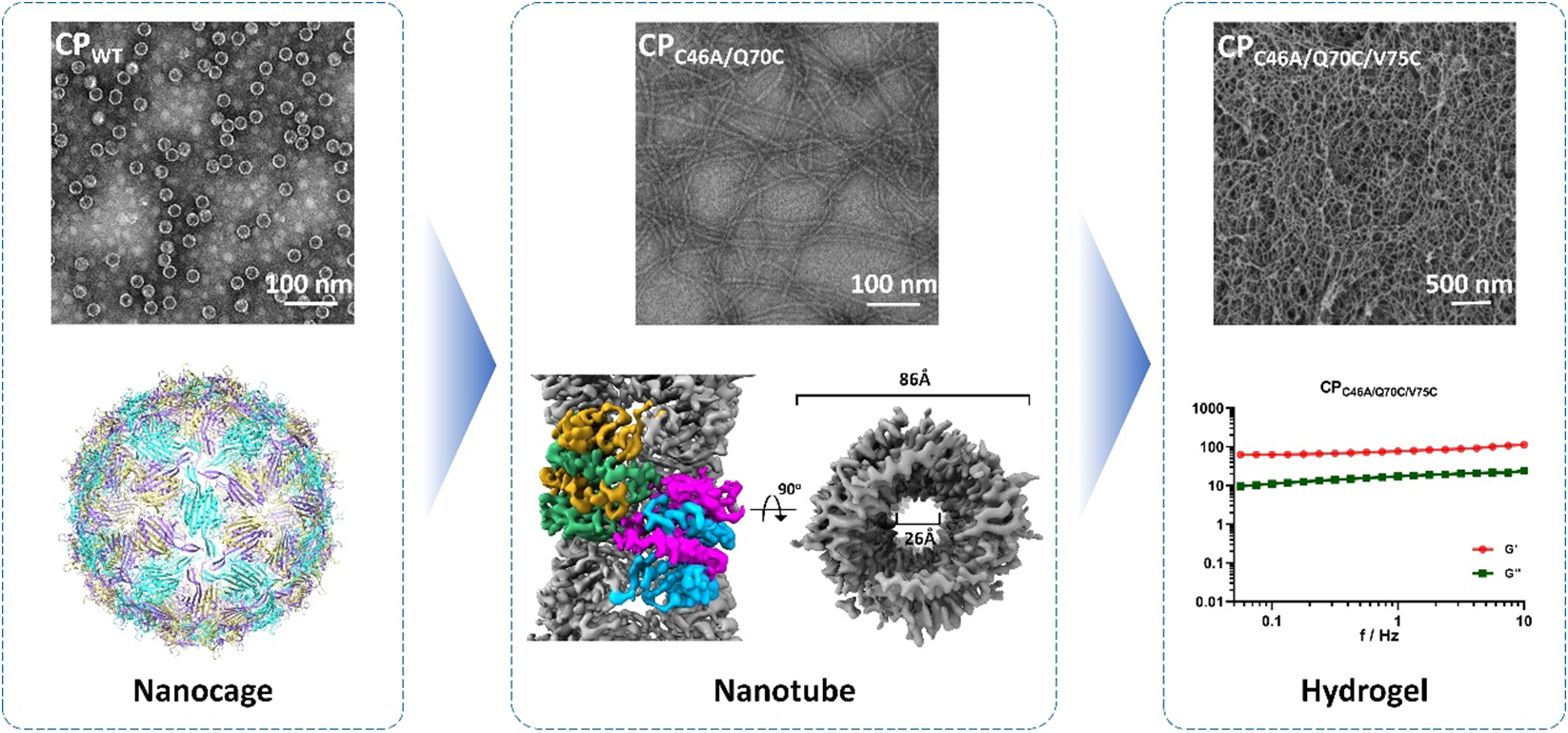
Figure 1. Morphological manipulation of MS2 CP assemblies from nanocages to nanotubes and hydrogels.
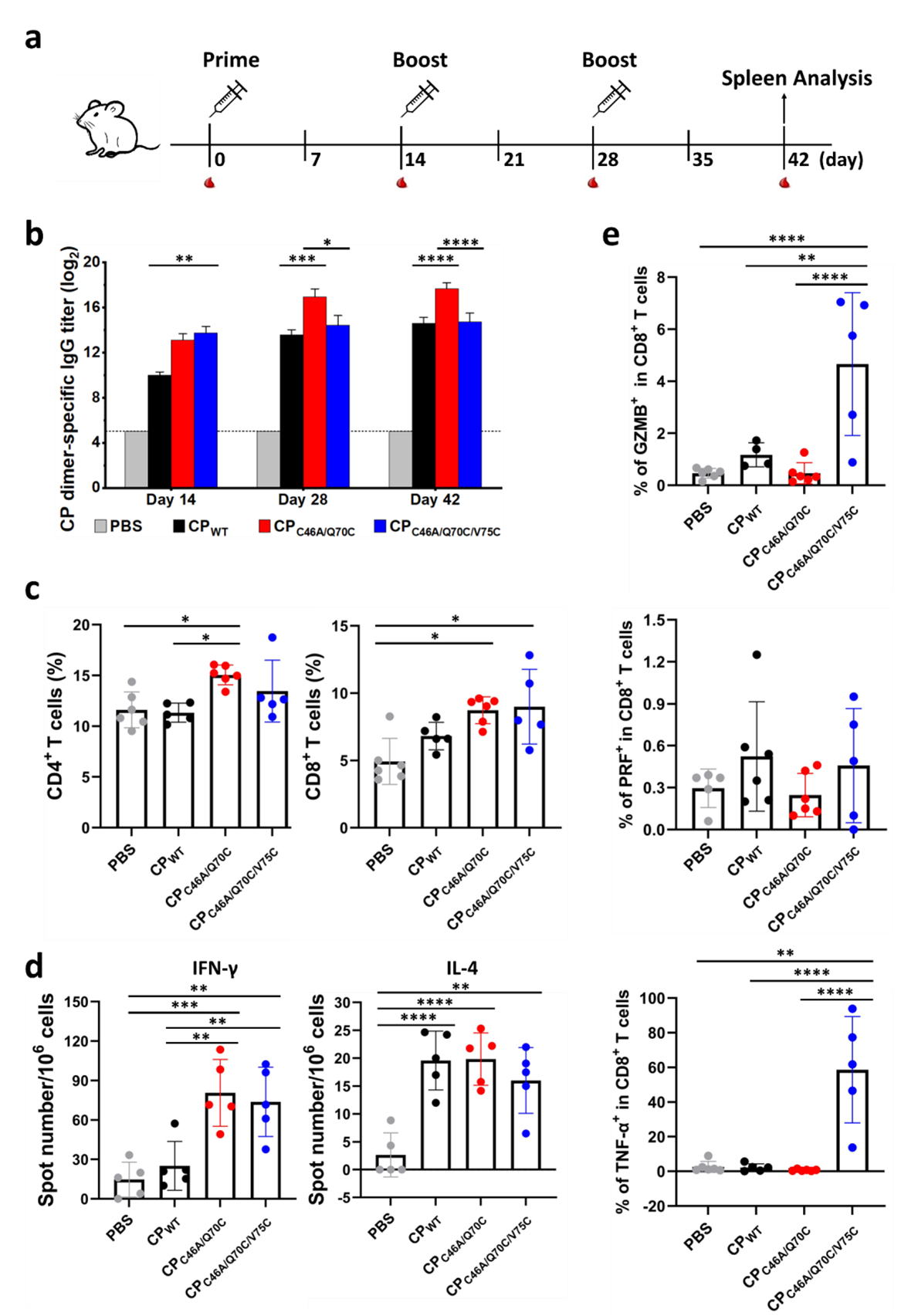
Figure 2. Impact of self-assembly morphology on the immune responses to MS2 CPs. CPWT, nanocages; CPC46A/Q70C, nanotubes; CPC46A/Q70C/V75C, hydrogels.