Researchers make new progress on the function of baculovirus PIF0 cleavage
Date:12-06-2024 | 【Print】 【close】
Baculoviruses enter insect midgut epithelial cells via a set of occlusion-derived virion (ODV) envelope proteins called per os infectivity factors (PIFs). To date, there are 10 PIF proteins identified, and nine of them (except for PIF5) form a PIF complex. Deleting any of these pif genes in the virus genome would result in total loss of viral per os infectivity. However, the working mechanisms of PIFs are largely unknown.
P74, as the first identified PIF, is also named PIF0. Previous studies found that P74 undergoes two sequential cleavages: the first cleavage is mediated by occlusion body endogenous proteinase when ODVs are released from occlusion body (OB) under alkaline conditions and the cleaved site of P74 is unknown, the second cleavage is mediated by host cell protease when the virus attaches to microvilli of the epithelial cells and the cleavage sites are R195/196/199. How these two sequential cleavages correlate to viral entry is not clear. Given the cleavage of viral envelope proteins is usually an important trigger for viral entry into host cells, dissecting the function and precise cleavage sites of P74 is necessary to understand the molecular mechanism of PIFs mediated virus entry.
In this study, the research group led by Prof. HU Zhihong from Wuhan Institute of Virology of the Chinese Academy of Sciences (CAS) uncovered that AcMNPV P74 is cleaved at R325 and R334 by proteinases either from OB or host epithelial cells. The previously reported second cleavage sites were not the real cleavage sites, simultaneous mutations in R195, R196, and R199 lead to instability of P74 during ODV release. Furthermore, the inevitable cleavage of P74 at sites R325 or R334 is likely to expose the potential fusion peptide at the N-terminus of the cleaved C-terminal P74. The mutation of R325/334 would result in significantly reduced oral infectivity. In conclusion, this study revised the cleavage model for P74 and implied that P74 might function as a class Ⅰ membrane fusion protein.
The study has been published in the Journal of Virology entitled “AcMNPV P74 is cleaved at R325 and R334 by proteinases of both OB and BBMV to expose a potential fusion peptide for oral infection”. This work was supported by the National Science Foundation of China and the Key Research Projects of Frontier Science, Chinese Academy of Sciences.
Paper link: https://journals.asm.org/doi/10.1128/jvi.00235-24
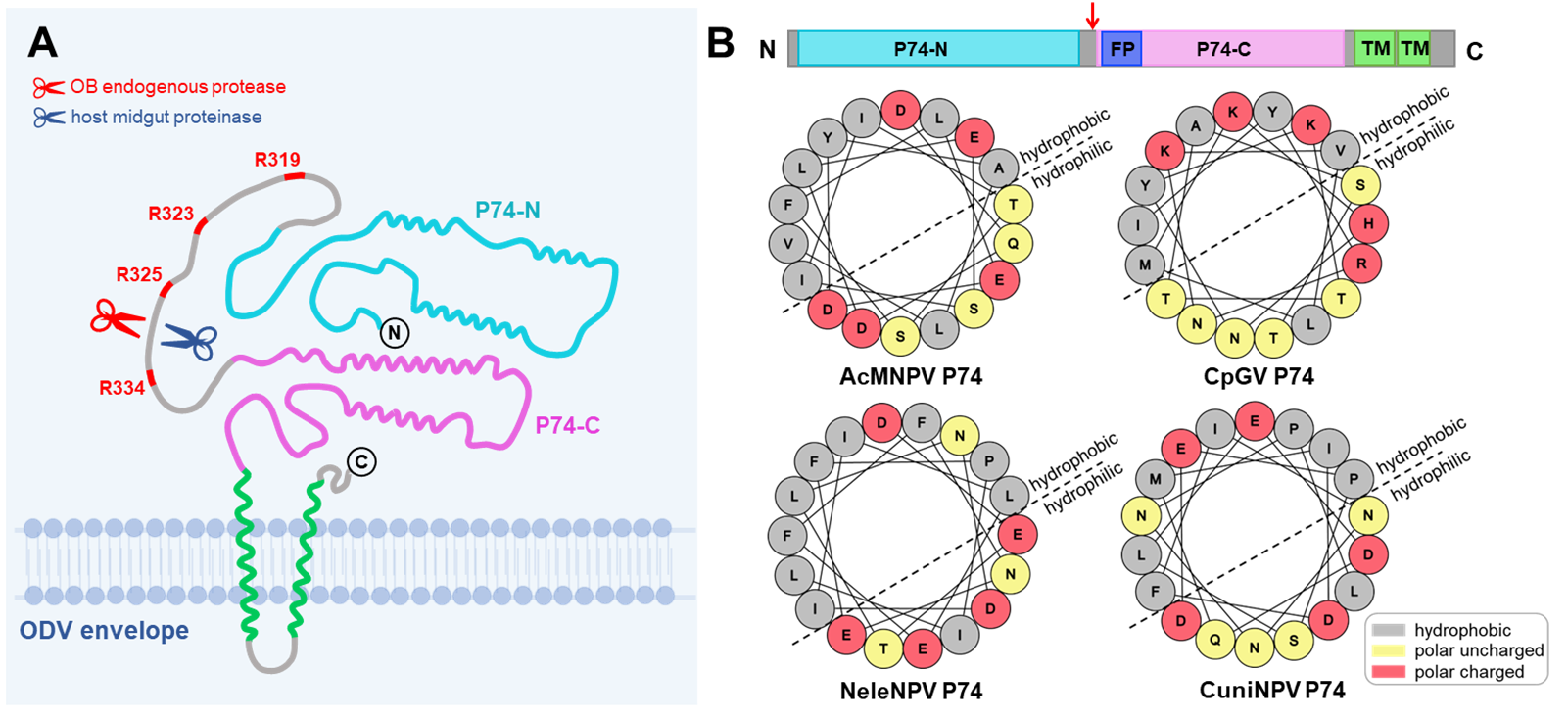
Figure legend: A. Revised cleavage model of P74; B. Cleavage of P74 exposes the potential fusion peptide at the N-terminus of P74-C